Abstract
Introduction: Treatment resistance remains a challenge that most patients (pts) with MM will encounter in their disease course. With each subsequent line of therapy (LOT), pt outcomes and health-related quality of life (HRQoL) worsen. Pts with MM who are refractory to immunomodulatory drugs, proteasome inhibitors (PIs), and anti-CD38 antibodies (triple-class refractory; TCR) have a poor prognosis and the holistic burden is not well understood. To capture these features, the ongoing prospective Connect ® MM Disease Registry was used to report treatment patterns and outcomes in pts with TCR MM.
Methods: Data from September 28, 2009 to February 4, 2021 were used. Eligible pts were ≥18 years of age at first documented diagnosis of MM, which became TCR during their disease course. MM was defined as refractory to therapies based on the International Myeloma Working Group Criteria. Index date was defined as first record of TCR during the disease course. Data analyzed included pt characteristics, treatment patterns pre- and post-index, overall survival (OS), healthcare resource utilization (HCRU), and HRQoL. General HRQoL was captured by EQ-5D-3L utility index (range −0.11-1, higher score indicated better HRQoL), EQ-5D visual analog scale (VAS) score (range 0-100, "worst imaginable" to "best imaginable" health status), and FACT-G total score (range 0-108). Disease-specific HRQoL was measured by FACT-MM total score (range 0-164), FACT-MM Trial Outcome Index (TOI) (range 0-112), and the Brief Pain Inventory (BPI) average pain item (range 0-10, "no pain" to "worst pain you can imagine"). Higher scores indicated better HRQoL for all FACT-related index scores. Cohort-level clinically meaningful change in HRQoL during post-index year 1 was defined as 0.08 for the EQ-5D utility index, 7 for the EQ-5D VAS, and 6-8 points for the FACT-G total score. Validated thresholds were unavailable for other index scores. All statistical analyses were descriptive.
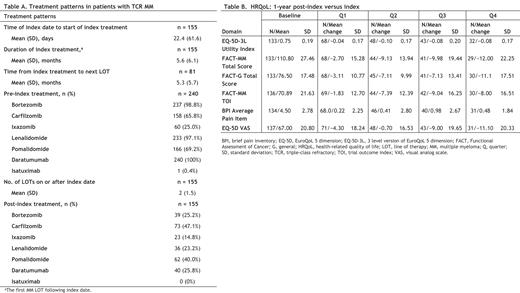
Results: This analysis included 240 pts identified from the Registry. Mean age at index date was 68.1 (standard deviation [SD] 10.4) years. Mean time from initial MM diagnosis was 64.1 (SD 26.9) months. 63.3% of pts had prior stem cell transplantation. Mean number of prior LOTs was 4 (SD 1.7). 64.6% of pts had received a subsequent MM LOT since index date. Median follow-up time post-index date was 4.6 months. Treatment pattern analysis of post-index treated pts (Table A) revealed that retreatment with an immunomodulatory drug, PI, or anti-CD38 agent post-index date was common, led by carfilzomib (47.1%) and pomalidomide (40.0%). However, no therapeutic combination was preferred in this pt population. Regarding their index therapy (first post-index LOT), 70.3% and 22.6% were on triplet and quadruplet combination therapy; 3.2% initiated belantamab mafodotin, selinexor, melflufen, and/or idecabtagene vicleucel; mean duration of treatment was 5.6 (SD 6.1) months. For all pts, median OS was 8.9 months; median OS for those who received < 4 prior LOTs (43.8%) versus those who received 4 or more prior LOTs (56.2%) was 10.0 and 8.0 months, respectively.
Regarding HCRU during the post-index follow-up, all-cause and MM-related hospitalizations were observed in 49.6% and 23.8% of all eligible pts, respectively. Among these pts, annualized mean number of all-cause/MM-related hospitalization and length of hospital stay were estimated as 8.5 (SD 18.2)/11.0 (SD 25.2) and 64.0 days (SD 80.0)/ 69.2 days (SD 83.7), respectively.
Analysis of HRQoL showed a numerical decline in HRQoL relative to index during the 1-year post-index period across all studied HRQoL instruments. There was a clinically meaningful decline in HRQoL from baseline following the index date based on the EQ-5D utility index, EQ-5D VAS, and FACT-G total score (Table B).
Conclusion: Pts with TCR MM experienced poor survival, substantial hospitalizations, and a clinically meaningful decline in HRQoL. 35.4% of pts did not receive any new LOTs since the disease became TCR. Among those who did receive subsequent LOTs, re-treatment with an immunomodulatory drug, PI, or anti-CD38 antibody therapy occurred, showing a lack of novel treatment options. This study suggests novel tolerable and efficacious therapeutic agents are needed to address the burden of illness in pts with MM.
Tang: Bristol-Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Hari: Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Celgene-BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Adaptive Biotech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Karyopharm: Consultancy; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Millenium: Membership on an entity's Board of Directors or advisory committees, Research Funding. Ramasamy: Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene - Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Weisel: Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy; Novartis: Honoraria; Pfizer: Honoraria. Joshi: Bristol Myers Squibb: Current Employment. Liu: Bristol Myers Squibb: Current Employment. Che: Bristol Myers Squibb: Current Employment. Hernandez: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Abonour: Jensen: Honoraria, Research Funding; Celgene-BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Research Funding; Takeda: Research Funding. Hardin: Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Rifkin: Sanofi: Membership on an entity's Board of Directors or advisory committees; McKesson: Current Employment, Current equity holder in publicly-traded company; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Fresenius-Kabi: Membership on an entity's Board of Directors or advisory committees; Coherus: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb (Celgene): Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. Ailawadhi: BeiGene, Ltd.: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Ascentage: Research Funding; Medimmune: Research Funding; Cellectar: Research Funding; Xencor: Research Funding; GSK: Consultancy, Research Funding; Takeda: Consultancy; Genentech: Consultancy; AbbVie: Consultancy; Karyopharm: Consultancy; Sanofi: Consultancy; Oncopeptides: Consultancy. Lee: Legend Biotech: Consultancy; Sanofi: Consultancy; Oncopetides: Consultancy; Bristol Myers Squibb: Consultancy; Amgen: Consultancy, Research Funding; Celgene: Consultancy; Janssen: Consultancy, Research Funding; Takeda Pharmaceuticals: Consultancy, Research Funding; Karyopharm: Consultancy; Regeneron: Research Funding; Genentech: Consultancy; GlaxoSmithKline: Consultancy, Research Funding. Terebelo: Pharmacylics: Speakers Bureau; Celgene-BMS: Consultancy; Takeda: Speakers Bureau; Janssen: Speakers Bureau. Durie: Amgen, Celgene/Bristol-Myers Squibb, Janssen, and Takeda: Consultancy; Amgen: Other: fees from non-CME/CE services . Narang: Bristol Myers Squibb: Consultancy; TG Pharma: Consultancy. Toomey: Bristol Myers Squibb: Consultancy. Gasparetto: Oncopeptide: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy; Sanofi: Consultancy, Honoraria, Speakers Bureau; Karyopharm: Consultancy, Honoraria, Speakers Bureau; Gsk: Consultancy, Honoraria, Speakers Bureau. Wagner: Celgene-BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Athenex Inc: Consultancy; Eli Lilly, Johnson & Johnson: Other: Spouse, previously held individual stocks;. Jagannath: Janssen Pharmaceuticals: Consultancy; Bristol Myers Squibb: Consultancy; Karyopharm Therapeutics: Consultancy; Legend Biotech: Consultancy; Sanofi: Consultancy; Takeda: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal